Fire extinguishing using liquid Nitrogen (LN2)
The advantages and practicality of fire extinguishing using liquid Nitrogen

Liquid Nitrogen (LN2)
Nitrogen makes up 78.08 per cent of the air volume and is colourless, odourless, and non-toxic. It is easily detachable from the air. Nitrogen is easily accessible as a byproduct of the oxygen generation process. When Nitrogen is cooled to 195.8 °C under normal pressure, it becomes liquid Nitrogen, with a density of 810 kg/m3.

Nitrogen is incombustible and has excellent stability. As a result, numerous advantages exist for utilising liquid Nitrogen as a fire extinguisher.
- Significant cooling capacity. Liquid Nitrogen has an exceptionally low temperature, typically 196 °C. As a result, liquid Nitrogen has a greater cooling capability. Liquid Nitrogen has a high capacity for heat absorption while rapidly vaporising. When 1 kilogramme of liquid Nitrogen fully vaporises at 0 °C, it may absorb 420 kJ of heat, forming a heat absorption barrier. It has the potential to lower the temperature of the combustion region and therefore weaken the flame. Nitrogen has a heat capacity of 1.038 kJ/(kgK) at constant pressure and 0.741 kJ/(kgK) at constant volume, implying it can also cool the zone as it is heated.
- Excellent suffocation effect. When the ambient temperature is 25 °C, liquid Nitrogen expands at a volume ratio of roughly 1:717. Liquid Nitrogen vaporises rapidly as it reaches the combustion region. Nitrogen’s volume percentage in the air rapidly increases, while oxygen’s volume fraction decreases. When the oxygen volume fraction in the space falls below roughly 15%, most plastics and solids, including wires and cables, become incapable of sustaining combustion, and the flame goes out.
- Capable of covering in the gaseous form. With a relative density of 0.98, liquid Nitrogen vaporises to become gaseous Nitrogen. Nitrogen spreads rapidly once released, gradually filling the region and rendering flammable gas inert. Thus allowing the nitrogen injection region to retain a low oxygen content for an extended period of time.
- Environmentally responsible. Nitrogen is one of the sufficient elements in the atmosphere and has zero global warming potential (GWP = 0). When discharged into the atmosphere during fire extinguishing, it has no adverse effect on the environment.
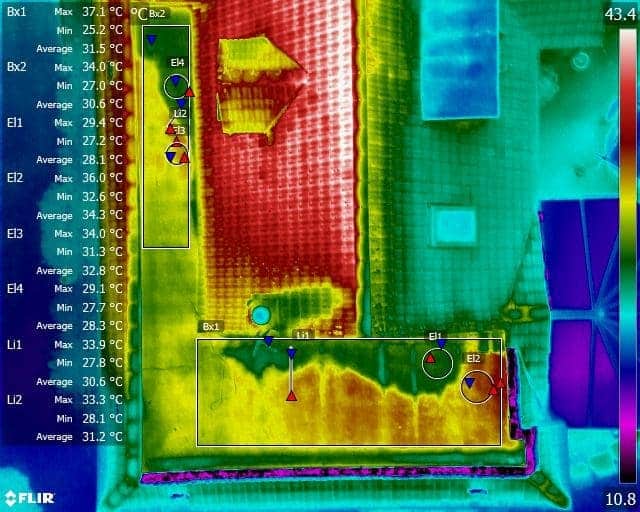
The Use Of Liquid Nitrogen following a Grain Store Explosion at the Port of Tilbury London
Following a Grain Store dust explosion at the Port Of Tilbury London in July 2020, the subsequent fire was managed by the pumping of Liquid Nitrogen into the grain silos to lower temperatures.
Drone Media Imaging provided the Port of Tilbury London and supporting emergency services with hourly, daily, and weekly calibrated thermal imagin reports on the temperature measurements that indicated the grain store fire’s development. Comprehensive reports, thermal imaging data, RGB photography, and video were utilised to efficiently convey the temperature facts necessary to handle the crisis until the building was successfully demolished in August 2021.
Aerial Thermography Services and Category 2 Thermographic Reporting
We are certified and approved drone pilots and work throughout the UK to provide both aerial and ground based photography, video/filming and thermography services. Fully insured and certified by the CAA with enhanced permissions for day and night time flights
Recent, Related and Associated Posts
The Importance of Solar Panel Inspections in Spring
Spring and summer are peak months for solar energy production—ensure your panels are operating efficiently with a professional thermal imaging inspection. Compliance with IEC62446-3:2017 is essential for both commercial and domestic systems, helping to prevent faults, optimise performance, and maintain insurance coverage. Book your inspection today!
In Thermography what is the difference between Quantitative vs Qualitative analysis
Quantitative and qualitative thermography are two essential methods in thermal imaging analysis. Quantitative thermography measures exact temperature values, while qualitative thermography focuses on pattern recognition. Both play a crucial role in building inspections, electrical fault detection, and industrial diagnostics. Understanding their differences helps professionals choose the right approach for accurate thermal assessments. Drone Media Imaging provides expert thermographic services, ensuring precise, reliable results. Contact us today for professional thermal imaging analysis.
Expert Infrared Inspections for Accurate Thermal Assessments
Need professional thermographic analysis for your project? Our certified experts use the latest infrared technology to deliver precise results. Contact Drone Media Imaging today for expert thermal imaging services.
related posts
Spring and summer are peak months for solar energy production—ensure your panels are operating efficiently with a professional thermal imaging inspection. Compliance with IEC62446-3:2017 is essential for both commercial and domestic systems, helping to prevent faults, optimise performance, and maintain insurance coverage. Book your inspection today!
Ensure your solar panels are spring-ready with these 5 essential steps: 1) Pre-inspection planning, 2) Drone setup and calibration, 3) Aerial thermal imaging scan, 4) Data analysis and interpretation, and 5) Post-inspection maintenance. Utilize thermal imaging drones for efficient, accurate, and safe solar panel inspections this spring.